By Margaret Kiss, PhD
Director of R&D
Ancera
As most people in the broiler industry know, necrotic enteritis (NE) is a devastating disease characterized by severe gut inflammation and sudden death, with mortality rates as high as 50% within a flock.
Economic losses due to NE extend far beyond mortality rates, however. Subclinical infection, characterized by extensive damage to the mucosal lining, leads to poor digestion and absorption of food, reduced weight gain and a decline in feed conversion.
The causative agent of NE is Clostridium perfringens, a gram-positive, anaerobic, spore-forming bacterium, which is part of the normal intestinal microflora of broiler chickens. The ability of the bacterium to cause disease depends on a variety of predisposing factors that create a favorable environment for C. perfringens to proliferate. Accordingly, the intestinal numbers of C. perfringens of healthy chickens compared to afflicted chickens can differ by several orders of magnitude.
New non-invasive approach to diagnosis
The rapid death caused by NE often prevents treatment of the disease. Conventional diagnosis requires an invasive procedure that involves a combination of bird dissection, Gram staining of mucosal scrapings and double zone hemolysis.
Because NE is associated with an increase in C. perfringens loads in the intestinal tract, a non-invasive method to monitor C. perfringens levels before clinical symptoms are observed could help in effectively preventing and treating the disease.
To determine whether non-invasive monitoring in fecal samples could detect an increase in C. perfringens levels in birds with subclinical or clinical infection, Ancera scientists conducted a pen trial at Ozark Avian Research in Gravette, Arkansas.
Using a model system, they challenged chickens with a virulent strain of C. perfringens, either alone or after pre-challenge with Eimeria maxima oocysts, which cause coccidiosis, to induce NE. Control groups received either antibiotics and an ionophore anticoccidial or a coccidiosis vaccine.
Investigators collected fecal samples for C. perfringens enumeration on scheduled days throughout the trial. They also performed lesion scoring on 25 birds in each group (out of 100 birds per pen) at the end of the 21-day challenge period.
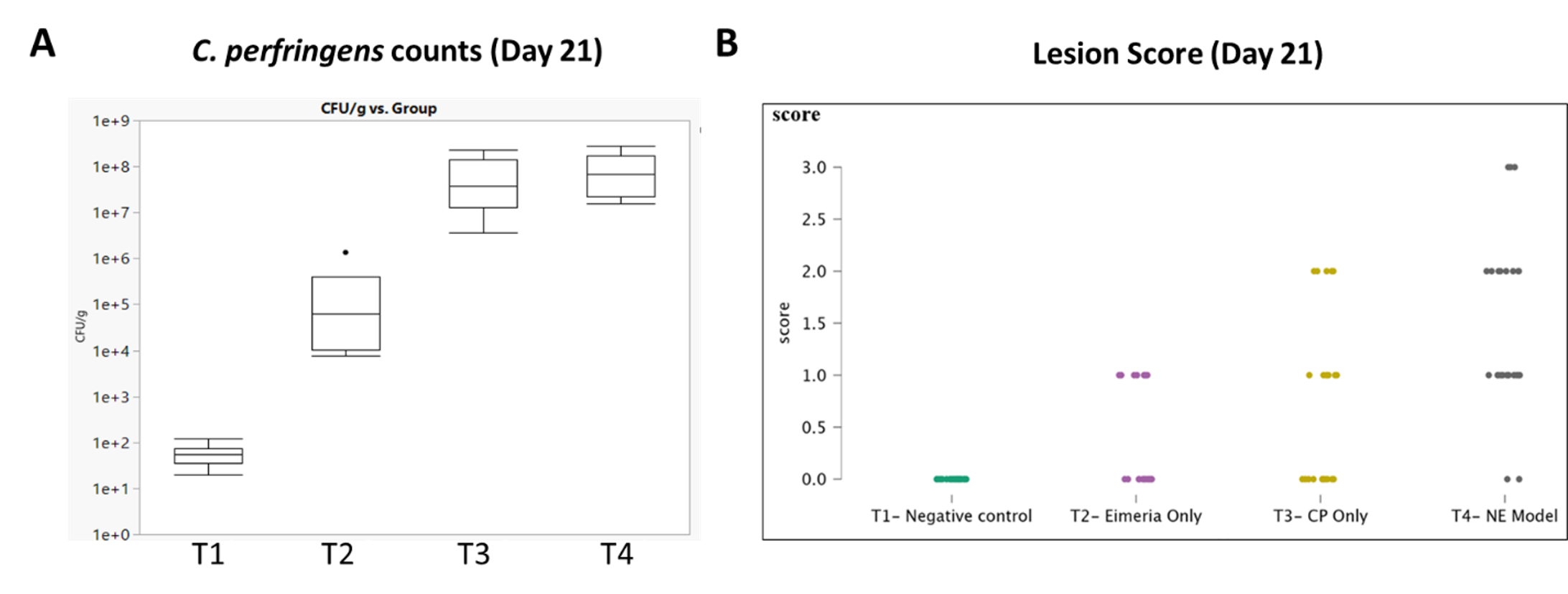
Although severe disease was not observed in the study, researchers observed higher lesion scores in C. perfringens-challenged birds both with and without E. maxima pre-challenge corroborated with an increase in average C. perfringens counts in fecal samples compared to the medicated or vaccinated birds (Figure 1).
Figure 1. Correlation between C. perfringens counts in feces and lesion score in birds
Investigators challenged 100 birds and were each given either antibiotics and an ionophore (T1), Coccivac®-B52 vaccine (T2), a virulent strain of C. perfringens (T3) or purified E. maxima oocysts followed by a virulent strain of C. perfringens (T4). The average C. perfringens counts in fecal samples from birds in treatment groups T3 and T4 were higher than in treatment groups T1 and T2 (A), which correlated with higher average lesion scores (B) in birds.
Ancera’s PIPER assay powers efficient monitoring at scale
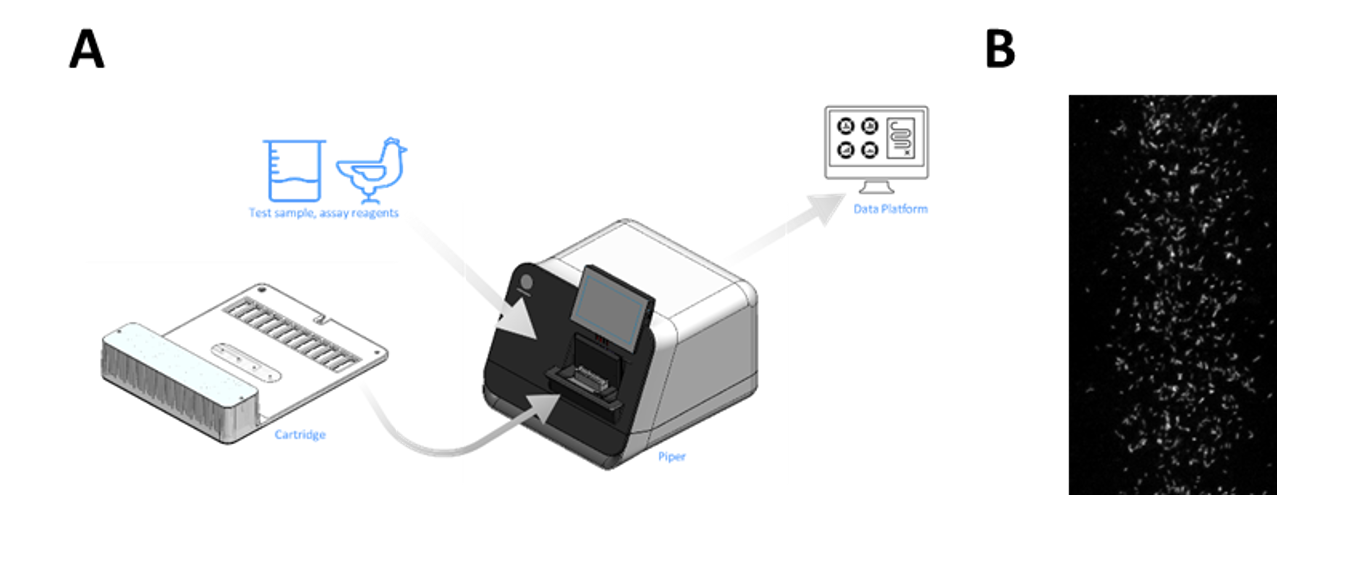
Conventional plate-based methods for analysis can be labor-intensive and time-consuming. To enable high-throughput sample monitoring, Ancera has applied proprietary, ferrofluid-driven, dynamic separation technology on the PIPERTM instrument — an advanced laboratory diagnostic workbench that combines cell-transport technology with molecular techniques for food production applications — to concentrate, image and count labeled cells of interest in a complex sample (Figure 2). The assay uses fluorescence in situ hybridization to distinguish between C. perfringens and other bacteria, including other Clostridium species.
All sample-processing steps are contained within a single-use cartridge, eliminating the potential for cross-contamination and need for routine instrument clean-up. Automated microscopy to identify and count labeled cells simplifies analysis and removes potential sources of operator error.
Unlike traditional flow cytometers. which are prone to clogging, PIPER does not require sample clean-up prior to loading it on the instrument. The entire assay, including specific labeling using fluorescent-labeled probe and analysis on PIPER, takes less than 3 hours from the time of sample receipt.
This assay can be paired with Ancera’s PIPER coccidia assay to measure oocyst levels and C. perfringens levels in the same flocks at the same time, providing a more granular understanding of the interplay between these two gut pathogens in the development of NE.
Figure 2. (A) Diagram of PIPER workflow and (B) PIPER image of labeled C. perfringens cells
NE compromises the health and welfare of poultry and the economic vitality of the poultry industry. Ancera’s non-invasive PIPER assay delivers preemptive disease identification and efficient monitoring at scale. To learn more, get in touch with an Ancera expert and start monitoring your flocks.
Editor’s note: Content on Modern Poultry’s Industry Insights pages is provided and/or commissioned by our sponsors, who assume full responsibility for its accuracy and compliance.