Fourth in a series
A successful coccidiosis-vaccination program starts with proper vaccine handling and uniform application at the hatchery. In the chicken house, uniform oocyst cycling will result in an immune response to protect against major Eimeria species responsible for coccidiosis in broilers with minimal to no post-vaccination reaction.
Of course, many factors can influence the final outcome, but there are numerous ways to monitor the bird to gain perspective on vaccine effectiveness.
“An important step to getting a coccidiosis-vaccination program off to the right start is to ensure chicks begin eating and drinking soon after placement,” says Charlie Broussard, DVM, Merck Animal Health. “Active chicks are more likely to access the sporulated oocysts they need to build immunity.”
He offers several areas to monitor to determine whether the vaccination program is on track to protect the flock.
1. Check crop fills
Despite providing adequate feed and water, some chicks do not eat or drink after placement. “Without proper monitoring, these ‘non-starter’ chicks may go undetected until it is too late, and the flock’s 7-day mortality spikes,” Broussard notes.
Plan to track the chicks’ eating and drinking habits early and often post-placement. An effective way to monitor this is to check the chicks’ crop fill at 24 and 48 hours after placement. He advises randomly selecting 100 chicks to examine their crops:
- A full, soft and rounded crop signals that the chick has found feed and water.
- A full but hard crop means the chick has found feed but not water.
- If the crop is empty, the chick has not found feed or water.
Crop-fill targets are 95% at 24 hours post-placement and 100% at 48 hours.
“If crop fills are below target levels, increase the number of supplemental feeders, increase light intensity and modify house temperature to improve chick comfort level,” Broussard says. “Also, walk the house to increase bird activity; they’ll start pecking and attract others to get up to eat and drink.”
2. Rectal temperatures and bird comfort
Monitoring rectal temperatures and evaluating bird behavior are both good methods to assess brooding conditions. “Huddling suggests chicks are cold; if chicks are excessively noisy and avoid supplemental heat, they are most likely hot,” Broussard notes.
Check rectal/vent temperatures within the first 48 hours of placement into the brood chamber of the house. He advises using a medical ear thermometer to monitor chicks by gently pushing up the tail to expose the vent and placing the thermometer tip on the bare skin of the vent area.
A target rectal/vent temperature range is between 103.2° F to 104.5° F (39.5° C to 40.3° C). Plan to adjust the environment, including adding or removing supplemental heaters, to meet the birds’ comfort needs.
3. Litter moisture — a delicate balance
Litter moisture plays a key role in uniform sporulated-oocyst cycling and is an indication of relative humidity in the house. But excessive moisture can increase sporulation, while producing overwhelming oocyst loads and other health-related challenges.
“There is a delicate balance to avoid litter moisture extremes of dry and dusty or wet and caked,” Broussard says.
A minimum of 25% litter moisture is needed for fecal droppings to remain moist long enough to achieve adequate oocyst sporulation. “Monitor litter moisture several times during the brooding period,” he advises.
To do this, form a ball with a handful of litter; if it temporarily holds its shape before falling apart, the litter moisture is adequate. If the litter remains in a ball, then moisture content is ≥ 35%, which may result in excessive sporulation and high oocyst levels.
4. Postmortem examination of vaccinated birds
The best method to determine what’s happening in the birds is to conduct postmortem examinations of vaccinated birds at varying ages. This will allow evaluation of coccidial cycling patterns and confirmation of expected immunity development.
For Coccivac®-B52, a vaccine used in broilers, Broussard suggests conducting postmortem exams prior to starting a vaccination program to better understand the current coccidiosis burden and potential carryover. The post-vaccination documentation should include gross lesions of Eimeria acervulina, Eimeria maxima, Eimeria tenella and Eimeria mivati, along with microscopic oocyst enumeration of E. maxima, to ensure each coccidial species falls within the expected lesion guidelines. “This also is an opportunity to evaluate overall bird health,” he says.
Once the vaccination program has begun, a health survey can be performed when the first vaccinated birds reach 28 days of age. Select birds that are 14 to 28 days old to evaluate gross lesions and microscopic evidence of E. maxima cycling. According to Broussard, evaluate at least two flocks of the same age at the following age ranges:
- < 17 days
- 18-21 days
- 22-24 days
- 25-28 days
“When a large enough sample is examined, it will provide a picture of coccidial cycling patterns,” he notes. “If the health survey shows irregular cycling patterns, a farm visit may be needed to check for abnormalities or management practices that are not compatible with controlled vaccine cycling.”
Subsequent, routine postmortem sessions should consist of vaccinated birds from days 14 through 35 (or longer for large birds). “The sampling guidelines for Coccivac-B52 are five birds per flock, with two to three flocks per interval age range to ensure accurate evaluation for expected cycling and immunity development,” Broussard says. Here are the flock age ranges:
- < 17 days
- 18-21 days
- 22-24 days
- 25-28 days
- 29-32 days
- 33-36 days
- 37-41 days
- 42-48 days
- 49 days to market
5. Coccidiosis-lesion index
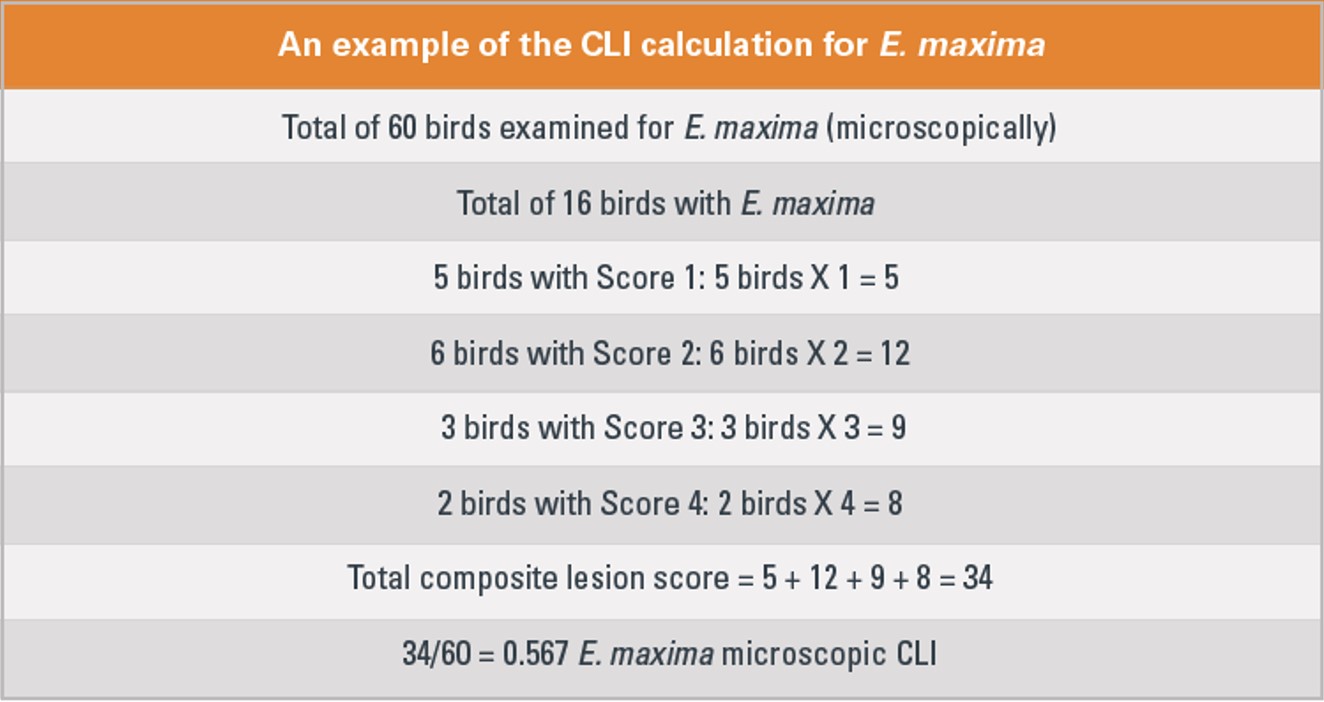
The coccidiosis-lesion index (CLI) provides the average coccidial score of all birds examined for each coccidia species. It is calculated by multiplying the numerical lesion scores (1 through 4) by the number of affected birds, then dividing by the total number of birds examined, Broussard points out (Figure 1).
A high CLI indicates high coccidial cycling levels in the birds. “The CLI may be a helpful value for evaluating vaccine performance over an extended period,” he adds.
Figure 1.
6. Anticoccidial-sensitivity testing
Anticoccidial-sensitivity testing (AST) is used to determine the coccidial resistance level or sensitivity loss to in-feed anticoccidials.
“An AST can be useful to demonstrate loss of efficacy by in-feed anticoccidials and whether an alternative control method such as a vaccine may be warranted to improve performance and restore sensitivity to the coccidial population in the chicken house,” Broussard points out.
For this process, oocysts are isolated and propagated from fresh, randomly collected fecal samples. Test groups are based on the in-feed products to be evaluated along with a positive and negative control.
Broussard explains that microscopically identified sporulated oocysts are quantified for inoculation into chickens at a specified age (10 to 14 days) at doses high enough to cause disease. After 6 days, the chickens are evaluated based on lesion scoring, bodyweight gain and feed conversion, with treated groups compared against inoculated controls.
“Information obtained from the AST can help determine which anticoccidials are more effective against the different Eimeria species,” he adds. “An AST also can be used to evaluate in-feed products used in conjunction with a coccidial vaccine, especially in antibiotic-free production systems.”
7. Oocysts per gram of feces
Another useful tool for monitoring any resistance development to in-feed anticoccidials or the onset of immunity is measuring oocysts per gram (OPG) of feces. This method involves collecting fresh fecal and cecal samples in random locations throughout the chicken house. This should be done about every 3 days to generate a coccidial oocyst-shedding curve, Broussard says.
“OPG can be used to track coccidiosis infections and determine when coccidial challenge is greatest,” he notes. “It also provides insight into coccidia cycling in vaccinated flocks without the need to euthanize birds.”
Broussard points out that Merck Technical Services can provide more information and guidance on setting up an AST or OPG-monitoring program.
Getting chicks vaccinated at the right time and dosage is just part of the equation; monitoring the effectiveness of that coccidial vaccination is an equally important part of the process.
For more tips and insights for managing coccidiosis, go here.
Other articles in this series:
Don’t overlook the basics when using coccidiosis vaccines
Coccidiosis vaccination: Are you creating an environment for success?
Managing feed, water critical for coccidiosis vaccination success
Coccidiosis vaccine checklist: Tips for maximizing performance
Editor’s note: Content on Modern Poultry’s Industry Insights pages is provided and/or commissioned by our sponsors, who assume full responsibility for its accuracy and compliance.