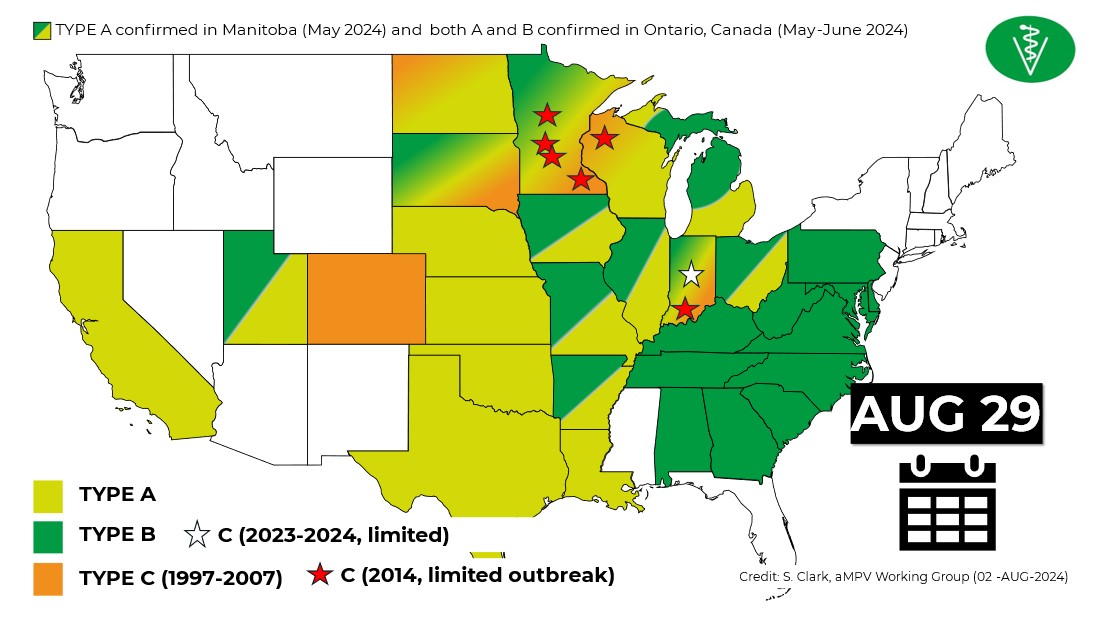
Avian metapneumovirus (aMPV) is now the leading health issue facing the US turkey industry, followed by the “lack of efficacious drugs for all diseases” and highly pathogenic avian influenza (HPAI), according to a recent survey of turkey industry professionals and veterinarians representing 73% of the 218 million birds raised in the US over a 12-month period ending August 2024.
The survey was conducted by the US Animal Health Association’s Committee on Poultry & Other Avian Species.
Surge of aMPV
The rapid emergence of aMPV dominated respondents’ concerns — and for good reason.
aMPV rose from the bottom of the 2023 survey results (No. 38) to the industry’s top concern after 2,355 cases were reported in US turkey flocks for the 12-month period ending August 31, 2024.
The disease was first reported in the US in late 2023. Subtype A was detected in California; subtype B was found in North Carolina.
“By January 2024, the rapid dissemination of aMPV was confirmed in both states and, within 4 months, it had spread to most poultry-producing regions nationwide,” said Steven Clark, DVM, DACPV, professional veterinary services manager at Huvepharma.
So far, aMPV has been detected only in commercial flocks. As part of epidemiology investigations, USDA’s Animal and Plant Health Inspection Service, Wildlife Services, tested 100 peridomestic species near aMPV-positive farms and did not find a single positive as of September 11, 2024, according to the agency’s David Marks.
In addition, of the 265 hunter-harvested migratory bird samples tested from four states (North Carolina, Virginia, South Carolina and West Virginia) by USDA’s Agricultural Research Service as of October 4, 2024, all were negative for aMPV A, B and C, according to David Surez, DVM, research leader at the agency.
Long-term impact of aMPV
One experience shared by a large US turkey company demonstrates the dramatic effects on flock mortality associated with aMPV. The company’s first suspected case of aMPV clinical disease occurred in November 2023. Over the next 3 months, average weekly company-wide flock mortality increased by 113% above the prior 12-month company average, with 1 week peaking at a 208% increase.
The effects of aMPV appear to be long-lasting. Following the introduction of aMPV, the company’s average weekly flock mortality remained 65% higher than pre-outbreak levels.1 Flock monitoring revealed that 98% of the company’s flocks are now infected with aMPV.
aMPV is not limited to turkeys, Clark said. In the US, all other categories of poultry — broiler chickens, egg layers and breeder poultry — have been infected. Turkeys are the most significantly impacted, however.
“Turkey breeders are experiencing egg-production declines ranging from 20% to 100%, lasting 2 to 4 weeks,” Clark reported. “This decrease in egg production during the initial outbreak contributed to a national shortage of poults.”
The Huvepharma veterinarian noted that in commercial turkey flocks, mortality rates are often severe, approaching 80% in some barns, with clinical disease persisting for up to 3 weeks.
Secondary infections, including Escherichia coli, cholera (Pasteurella multocida), ORT (Ornithobacterium rhinotracheale) and MG (Mycoplasma gallisepticum), complicate the clinical disease in all poultry species.
“The poultry industry urgently requires both live and killed (inactivated) vaccines for aMPV subtypes A and B for both chickens and turkeys,” Clark added.
aMPV vaccines
As of September 28, 2024, there are USDA-approved live vaccines for aMPV and only four special approvals for inactivated (killed) vaccines.
Two inactivated vaccines (Hipra and Boehringer Ingelheim) were granted import permits by USDA; two other vaccine manufacturers (Cambridge Laboratories/Merck and Ceva) are domestically producing experimental autogenous vaccines, with US-origin subtype B isolates, to control outbreaks of aMPV in poultry farms in the US.
“The ongoing spread of aMPV across major poultry-producing regions in the US and Canada highlights the need for immediate and effective vaccination strategies,” Clark said.
“The disease continues to circulate among poultry in all the states, although cycling between low and high mortality rates, especially in turkey flocks. Regulatory bodies are encouraged to expedite the review and approval of suitable vaccines, in collaboration with industry’s development, to mitigate the severe impacts on poultry health and production.
Other industry concerns
The “lack of approved, efficacious drugs” and other government-approved preventives and therapies remains a major obstacle to efficient turkey production in the US. It is likely a contributor to all turkey-health challenges and disease issues facing the industry, Clark said.
There is still no vaccine or treatment for blackhead disease.
The veterinarian also pointed to recurring challenges with coccidiosis. An effective coccidiosis-control program in turkeys involves the use of anticoccidial medications, or live vaccines and the subsequent development of immunity.
“Some production programs do not permit the use of antibiotics, including ionophore anticoccidials, and some programs prohibit FDA-approved chemical anticoccidials,” Clark said. “Anticoccidial programs therefore rely on vaccination.”
Currently, Clark reported, there are two commercial live vaccines for turkey coccidiosis. In 2024, USDA granted conditional approval to a three-strain commercial turkey coccidiosis live vaccine (Huvepharma) to complement a previously licensed two-strain product (Ceva). Table 4 summarizes the US turkey production coccidia-control programs.
To access the full results of the survey with additional analysis, click here.
Table 1. Turkey health survey (August 2023-2024) of professionals in US turkey production (n = 21, head reporting = >159.2 million) ranking current disease issues (1 = no issue to 5 = severe problem). Data on file.
| Issue | Score average (1-5) |
| Avian Metapneumovirus (aMPV) | 4.9 |
| Lack of approved, efficacious drugs | 4.8 |
| Avian Influenza, High Path (HPAI) | 4.2 |
| Colibacillosis | 4.0 |
| Clostridial Dermatitis (Cellulitis) | 3.9 |
| TR-DFTR (Turkey Reovirus Digital Flexor Tendon Rupture) | 3.7 |
| Ornithobacterium rhinotracheale (ORT) | 3.4 |
| Salmonella | 3.3 |
| THRV (Turkey Hepatitis Reovirus) | 3.3 |
| Leg Problems | 2.8 |
| Bordetella avium | 2.5 |
| Late Mortality | 2.5 |
| Blackhead (Histomoniasis) | 2.4 |
| Cholera | 2.4 |
| Heat Stress/Mortality | 2.3 |
| Coccidiosis | 2.2 |
| Tibial Dyschondroplasia (TDC, Osteochondrosis) | 2.2 |
| Bleeders (aortic, hepatic ruptures) | 2.1 |
| Mycoplasma synoviae (MS) | 2.1 |
| Cannibalism | 2.0 |
| Poult Enteritis of unknown etiologies | 2.0 |
| Protozoal Enteritis (Flagellated) | 2.0 |
| Streptococcus gallolyticus (aka, S. bovis) | 2.0 |
| Breast Blisters and Breast Buttons | 2.0 |
| Avian Influenza, Low Path (LPAI) | 1.8 |
| Osteomyelitis (OM) | 1.8 |
| Round Worms (Ascaridia dissimilis) | 1.8 |
| Newcastle Disease Virus (NDV) | 1.8 |
| H3N2 (H1N1) Swine Influenza | 1.7 |
| Necrotic enteritis | 1.7 |
| Turkey Coronavirus | 1.6 |
| Mycoplasma gallisepticum (MG) | 1.6 |
| PEMS (Poult Enteritis Mortality Syndrome) | 1.5 |
| Shaky Leg Syndrome | 1.4 |
| Fractures | 1.4 |
| Erysipelas | 1.1 |
| Mycoplasma meleagridis (MM) | 1.0 |
| Mycoplasma iowae (MI) | 1.0 |
| Spondylolisthesis (Kinky-Back) | 1.0 |
Table 1A. Turkey health survey (August 2023-2024) of professionals in US turkey production (n = 21, head reporting = >159.2 million): Enteric diseases ranking for 2024.
| Issue | Score Average (1-5) | Overall Rank (1-39) |
| Blackhead (Histomoniasis) | 2.4 | 13 |
| Coccidiosis | 2.2 | 16 |
| Poult Enteritis of unknown etiologies | 2.0 | 21 |
| Protozoal Enteritis (Flagellated) | 2.0 | 22 |
| Round Worms (Ascaridia dissimilis) | 1.8 | 27 |
| Necrotic enteritis | 1.7 | 30 |
| Turkey Coronavirus | 1.6 | 31 |
| PEMS (Poult Enteritis Mortality Syndrome) | 1.5 | 33 |
Table 1B. Turkey health survey (August 2023-2024) of professionals in US turkey production (n = 21, head reporting = >159.2 million): Respiratory diseases ranking for 2024.
| Issue | Score Average (1-5) | Overall Rank (1-39) |
| Avian Metapneumovirus (aMPV) | 4.9 | 1 |
| Avian Influenza, High Path (HPAI) | 4.2 | 3 |
| Colibacillosis | 4.0 | 4 |
| Ornithobacterium rhinotracheale (ORT) | 3.4 | 7 |
| Bordetella avium | 2.5 | 11 |
| Cholera | 2.4 | 14 |
| Mycoplasma synoviae (MS) | 2.1 | 19 |
| Avian Influenza, Low Path (LPAI) | 1.8 | 25 |
| Newcastle Disease Virus (NDV) | 1.8 | 28 |
| H3N2 (H1N1) Swine Influenza | 1.7 | 29 |
| Mycoplasma gallisepticum (MG) | 1.6 | 32 |
Table 2. Turkey health survey (August 2023-2024) of professionals in US turkey production (n = 20, head reporting = 159.2 million): Reporting cases of diseases. Data on file.
| Cases (##) of | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 |
| Blackhead (Histomoniasis) | 51 | 61 | 103 | 130 | 82 | 96 |
| Mycoplasma synoviae (MS) | 161 | 20 | 14 | 34 | 21 | 25 |
| Turkey Coronavirus (TCV) | 31 | 411 | 459 | 117 | 27 | 95 |
| Turkey Reovirus Digital Flexor Tendon Rupture | 368 | 487 | 170 | 239 | 548 | 486 |
| Mycoplasma gallisepticum (MG) | 43 | 12 | 8 | 78 | 31 | 30 |
| Avian Metapneumovirus (aMPV) | 2355 | – | – | – | – | – |
Table 3. Turkey health survey (August 2023-2024) of professionals in US turkey production (n = 20, head reporting = 159.2 million) by antibiotic program. Data on file.
| 2024 | 2023 | |
| Conventional/Full Use1 | 56% | 59% |
| No growth promotants, CRAU/CRAU-like2 | 25% | 23% |
| NAE/ABF, RWA3 | 19% | 18% |
1Conventional/Full Use (any antibiotics, including ionophores, bacitracin, bambermycins, and /or those deemed medically important to humans by FDA), allows in-feed and in-water administration of antibiotics.
2No Growth Promotants, CRAU/CRAU-like (Certified Responsible Antibiotic Use), permits only therapeutic uses.
3No Antibiotics Ever (NAE) /Antibiotic Free (ABF), Raised Without Antibiotics (RWA), does not use neither in-feed nor in-water antibiotics. No hatchery injection of antibiotics.
Table 4. Turkey survey (August 2023-2024) of professionals in US turkey production (n = 20, head reporting = 159.2 million) coccidia-control programs. Does not total 100%. Alternatives (phytonutrients) and vaccines may be used to supplement the current ionophore or chemical anticoccidial program, or as the sole program for coccidia control. Data on file.
| Program | 2024 | 2023 |
| Ionophore | 56% | 59% |
| Chemical | 41% | 36% |
| Alternative (phytonutrients) | 11% | 18% |
| Vaccine | 26% | 14% |
Figure 1: Map of aMPV cases in poultry (Clark, aMPV Working Group)
Editor’s note: Content on Modern Poultry’s Industry Insights pages is provided and/or commissioned by our sponsors, who assume full responsibility for its accuracy and compliance.
1 Nov 29, 2023 – Feb 26, 2024, average weekly company-wide flock mortality was 1.28%, and the prior 12-month company (No 2022 – Nov 2023) average was 0.60%, and week of Jan 1, 2024, peaked at 1.85%. Following the winter introduction, average weekly flock mortality is 0.99% (March – September 2024) compared to before the disease introduction in 2023.